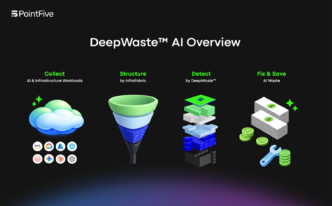
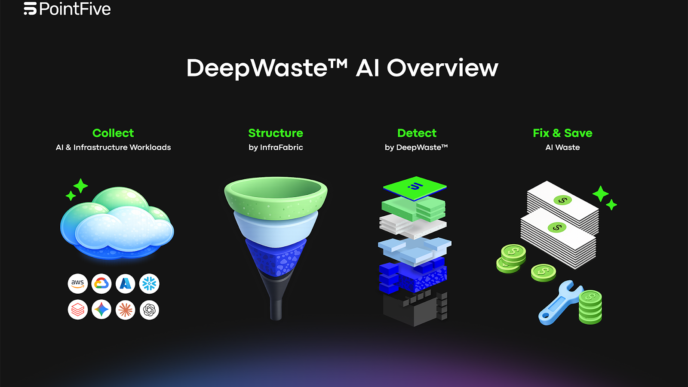
So, Field Medical just got a big boost, snagging $35 million in Series B funding. This money is all about pushing forward their pulsed field ablation tech, which sounds pretty neat for heart issues. They’ve already raised a good chunk of cash before this, hitting $75 million total since they started. It looks like they’re really trying to make a difference in how doctors treat heart rhythm problems, especially for folks with more serious conditions.
Key Takeaways
- Field Medical secured $35 million in an oversubscribed Series B funding round, with total funding now at $75 million.
- The new capital will speed up the development of their FieldForce™ Pulsed Field Ablation platform and the VERITAS clinical trial.
- Their technology uses ultra-low energy, pulsed fields to target cardiac tissue, aiming for greater safety and precision than older methods.
- The company is focused on treating challenging conditions like high-risk ventricular tachycardia and atrial fibrillation.
- Field Medical has gained FDA Breakthrough Device Designation and is part of the FDA’s TAP Pilot Program, moving towards market availability.
Field Medical Secures Significant Series B Funding
Oversubscribed Round Accelerates Pulsed Field Ablation Innovation
Field Medical just announced they’ve closed a $35 million Series B funding round. This wasn’t just any funding round; it was oversubscribed, meaning more investors wanted in than there was space for. That’s a pretty good sign investors are excited about what Field Medical is doing. This new money is going straight into speeding up their work on pulsed field ablation (PFA) technology, especially their FieldForce™ system. They’re planning to push forward with their VERITAS clinical trial, which is a big deal for getting their technology out there.
New Capital Fuels VERITAS Clinical Trial Advancement
The $35 million injection is a major boost for the VERITAS trial. This study is key to showing how well their PFA system works, particularly for patients dealing with high-risk ventricular tachycardia (VT). It’s a tough condition to treat, and current options aren’t always ideal. Field Medical’s approach aims to be safer and more precise than older methods, which is exactly what doctors and patients are looking for. This funding means they can really get the trial moving and gather the data needed to show the system’s potential.
Total Funding Reaches $75 Million Since Founding
With this latest $35 million Series B, Field Medical has now raised a total of $75 million since they started back in 2022. That’s a lot of money in a short amount of time. It shows a lot of confidence from investors in their vision for changing how cardiac ablation is done. This kind of backing is pretty important for a company trying to bring a new medical technology to market, especially one that could make a real difference for people with heart rhythm problems.
Field Medical’s Pulsed Field Ablation Technology
Proprietary FieldForce™ Platform Enhances Safety and Precision
Field Medical is really pushing the envelope with their FieldForce™ platform. It’s built around pulsed field ablation (PFA), which is a pretty neat way to target heart tissue. Instead of using heat like older methods, PFA uses short bursts of electrical energy. The big deal here is that it’s supposed to be way more precise. This means it can zap the bad tissue without messing up the important stuff nearby, like nerves or the esophagus. That’s a huge win for patient safety.
Ultra-Low Energy System Targets Cardiac Tissue Selectively
What’s interesting about Field Medical’s system is how little energy it uses. They’ve managed to make it work with really low energy levels, which is key to that selective targeting we just talked about. It’s like using a laser pointer instead of a floodlight. This approach is designed to create lesions in the heart muscle that stop irregular heartbeats, but it’s supposed to leave the surrounding structures unharmed. This is a big step up from thermal ablation, which can sometimes cause collateral damage.
FieldBending™ Technology Shapes Electric Fields for Optimal Ablation
They’ve also got this thing called FieldBending™ technology. Basically, it lets them control the shape of the electrical field that the PFA system creates. Think of it like being able to sculpt the energy. This allows them to really fine-tune how the ablation happens, making sure it’s effective and safe. It’s a pretty clever way to get the best results from the pulsed electrical energy. It’s all about making the treatment as good as it can be for the patient.
The goal is to make cardiac ablation safer and more effective by using electrical pulses that are kinder to the body’s non-target tissues. This technology aims to reduce the risks associated with current treatments, offering a better option for people with heart rhythm problems.
Addressing Unmet Needs in Cardiac Care
Focus on High-Risk Ventricular Tachycardia Treatment
Ventricular tachycardia (VT) is a really tough one for doctors to treat. It’s a fast heart rhythm that can be life-threatening, and honestly, current tools aren’t always cutting it. Many patients who suffer from VT are already dealing with other serious heart issues, making the situation even more delicate. Field Medical is zeroing in on this problem with their pulsed field ablation (PFA) technology. They’re aiming to create a more precise way to stop these dangerous rhythms without causing extra damage. This focus on VT is a big deal because it tackles a condition where treatment options are limited and the stakes are incredibly high.
Improving Outcomes for Atrial Fibrillation Patients
While VT is a major focus, the technology also holds promise for atrial fibrillation (AFib). AFib is much more common, and while treatments exist, they aren’t perfect. Some patients don’t respond well to medication, and traditional ablation methods, which use heat or cold, can sometimes affect nearby important structures like nerves or the esophagus. Field Medical’s PFA system uses electrical pulses instead of heat, which could mean a gentler approach. The idea is to zap the problematic heart tissue causing AFib while leaving the surrounding areas unharmed. This could lead to fewer complications and better results for a lot of people.
Reducing Complications Compared to Thermal Ablation
Traditional ablation methods, like radiofrequency or cryoablation, have been around for a while and work by heating or freezing tissue to create scars that block abnormal electrical signals. But, this can be a bit of a blunt instrument. There’s always a risk of collateral damage to things like the phrenic nerve (which helps you breathe) or the esophagus. PFA, on the other hand, is designed to be much more selective. It uses short bursts of electricity that essentially create tiny holes in the cell membranes of the target tissue, causing it to die off. The hope is that this electrical approach will significantly lower the chances of those scary complications, making the whole procedure safer for patients.
Strategic Growth and Regulatory Milestones
FDA Breakthrough Device Designation Achieved
Getting the FDA’s Breakthrough Device Designation is a pretty big deal. It means the agency sees Field Medical’s pulsed field ablation (PFA) technology as potentially offering a significant improvement over existing treatments for serious conditions like ventricular tachycardia. This designation isn’t just a label; it comes with a commitment from the FDA to work closely with the company, speeding up the review process and providing guidance. It’s a clear signal that the technology is on the right track for addressing unmet needs in cardiac care.
Selection for FDA’s TAP Pilot Program
Field Medical’s inclusion in the FDA’s Total Product Lifecycle Advisory Program (TAP) Pilot Program is another major step. This program is designed to help innovative medical devices get to patients faster by offering early and frequent communication with the FDA throughout the development and review stages. Being selected suggests the FDA recognizes the potential of Field Medical’s PFA system and wants to support its journey toward market approval. It’s a collaborative effort aimed at ironing out any regulatory kinks early on.
Advancing Towards Commercialization
With the recent $35 million Series B funding, Field Medical is really gearing up for the next phase. This capital injection is specifically earmarked to push forward the VERITAS clinical trial, which is key to gathering the data needed for regulatory approval. The company is also building out its clinical and regulatory teams, getting everything in place to not only complete trials but also prepare for eventual market launch. It feels like they’re moving with real purpose, aiming to bring this new ablation method to doctors and patients sooner rather than later.
The journey from a promising technology to a widely adopted medical treatment is complex. Field Medical’s strategic focus on obtaining regulatory designations and participating in FDA pilot programs demonstrates a clear understanding of this path. These steps are not just about meeting requirements; they are about building confidence with regulators and paving the way for broader access to potentially life-changing therapies.
Strengthening Field Medical’s Leadership
Expansion of Scientific Advisory Board
Field Medical isn’t just about the tech; they’re building a powerhouse team to back it up. They’ve recently beefed up their Scientific Advisory Board, bringing in some seriously smart folks who know cardiac care inside and out. Think of them as the seasoned guides helping steer the ship.
Addition of Prominent Electrophysiology Experts
Specifically, they’ve added a few big names from the world of electrophysiology. These aren’t just any doctors; they’re people who have spent years working with patients who have heart rhythm issues and understand the real-world challenges. Having their insights is pretty key for making sure the technology actually works well for both doctors and patients.
New Executive Team Members Drive Scale-Up
On top of the advisors, Field Medical has also brought in new people to lead the executive team. These are folks with experience in growing medical device companies. It signals that they’re not just focused on developing the technology but are also getting ready to make it available more widely. It’s like getting the right people in place before a big product launch.
Building a strong leadership team, both in terms of scientific guidance and business operations, is just as important as the innovation itself. It shows a commitment to not only developing a new treatment but also to seeing it through to widespread adoption and patient benefit.
Here’s a look at some of the key additions:
- Dr. Alexei Mlodinow, MD, MBA: Brings a wealth of clinical and business acumen.
- Marlou Janssen: Former President of Biotronik U.S., offering extensive medtech market experience.
- Mark Wisniewski: Named Chairperson, bringing financial leadership from his CFO role at Enterra Medical.
These moves suggest Field Medical is serious about scaling up and making a real impact on how heart rhythm problems are treated.
Future of Arrhythmia Treatment
Redefining Safety and Efficacy Standards
Field Medical is really trying to change the game when it comes to treating heart rhythm problems. They’re focused on making sure treatments are not only effective but also super safe, which is a big deal for patients. The goal is to set a new bar for what doctors expect from ablation procedures. It’s all about giving patients better outcomes with fewer worries.
Positioned to Impact Cardiac Ablation Care
With the new funding and their unique technology, Field Medical is in a good spot to make a real difference. They’re looking at how to improve treatments for things like ventricular tachycardia, which can be really tough to manage. The idea is to offer something that works better and is less risky than what’s available now. It feels like a step forward for everyone involved in cardiac care.
Offering Safer, Faster, Targeted Solutions
What’s really interesting is how Field Medical’s technology works. It uses pulsed electrical fields instead of heat, which could mean less damage to healthy tissue around the heart. This approach aims to be:
- Safer: By avoiding heat, they hope to reduce the chances of harming important nearby structures like nerves or the esophagus.
- Faster: The procedures might become quicker, meaning less time in the operating room for patients.
- Targeted: The technology is designed to focus precisely on the areas of the heart that need treatment.
The push for new ways to treat heart arrhythmias is driven by a clear need for options that are both highly effective and minimize the risks associated with current methods. Field Medical’s approach with pulsed field ablation seems to directly address these concerns, aiming for a gentler yet powerful way to restore normal heart rhythms.
It’s exciting to think about how this could change things for people dealing with heart rhythm issues. The focus on safety and precision is something many patients and doctors will likely appreciate. We’ll have to see how it all plays out in the coming trials and eventually in clinics, but the potential is definitely there.
Looking Ahead
So, Field Medical just landed a pretty big chunk of change – $35 million, to be exact. This cash injection is all about pushing their pulsed field ablation tech forward, especially for tough heart rhythm issues like VT and AFib. They’re aiming to make treatments safer and work better than what’s out there now, which is great news for patients. With this funding, they can really get their VERITAS trial rolling and keep improving their FieldForce system. It seems like they’re building something that could really change how doctors treat these conditions, offering a new, potentially less risky option for people dealing with heart rhythm problems.