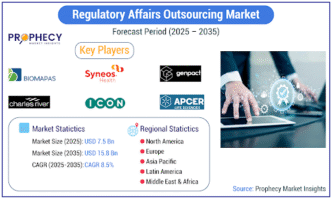
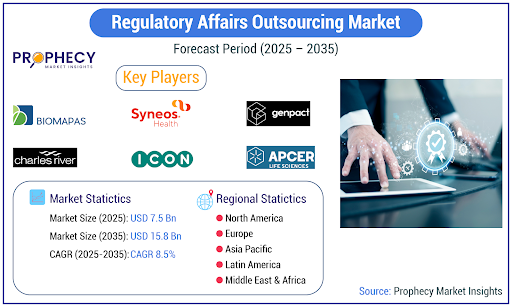
The Regulatory Affairs Outsourcing (RAO) market is experiencing a robust period of growth, driven by an increasingly intricate global regulatory landscape and the strategic imperative for life sciences companies to optimize costs and leverage specialized expertise. According to the latest analysis, the market, which was valued at USD 7.1 Billion in 2024, is anticipated to enlarge at a rate reaching USD 15.8 Billion by 2035, exhibiting a Compound Annual Growth Rate (CAGR) of 8.5% during the forecast period.
This substantial expansion underscores a significant trend within the pharmaceutical, biotechnology, and medical device sectors: a growing reliance on external partners to navigate the complexities of regulatory compliance. Companies are increasingly recognizing the value of outsourcing these critical functions to dedicated service providers who possess deep regulatory knowledge and technological capabilities.
Key Drivers Fueling Market Acceleration:
The surge in the RAO market is primarily attributed to several interconnected factors:
- Heightened Regulatory Complexity and Stringency: Regulatory frameworks worldwide are becoming more intricate and demanding, encompassing new guidelines for drug development, medical device approvals, and post-market surveillance. This escalating complexity necessitates specialized expertise that in-house teams may struggle to maintain, driving the demand for external support.
- Globalization of Life Sciences Operations: As pharmaceutical and medical device companies expand their footprint into diverse international markets, they encounter a myriad of regional and national regulatory requirements. Outsourcing provides a streamlined and cost-effective solution for managing these disparate compliance landscapes, facilitating smoother global market entry.
- Intensified R&D Activities and Clinical Trials: A continuous increase in research and development initiatives, coupled with a rising number of clinical trial applications and new product registrations, generates a substantial volume of regulatory work. RAO providers are crucial in managing these workloads efficiently and ensuring timely submissions.
- Strategic Focus on Core Competencies and Cost Efficiency: Companies are increasingly opting to outsource non-core functions like regulatory affairs to concentrate their internal resources on primary activities such as innovation, research, and manufacturing. This strategic shift not only enhances focus but also leads to significant cost savings by reducing overheads associated with in-house regulatory departments.
- Technological Advancements in Regulatory Processes: The integration of advanced technologies, including Regulatory Information Management Systems (RIMS), artificial intelligence (AI), and machine learning, is revolutionizing regulatory workflows. Outsourcing providers often lead the adoption of these technologies, offering clients enhanced efficiency, accuracy, and faster turnaround times for submissions and compliance tasks.
📥 Download the sample report here
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/1114
Navigating Challenges and Seizing Opportunities:
While the outlook for the RAO market is overwhelmingly positive, certain challenges persist:
- Data Security and Privacy Concerns: The handling of highly sensitive intellectual property, clinical data, and patient information by third-party providers raises critical data security and privacy concerns. Ensuring robust cybersecurity protocols and adherence to stringent data protection regulations is paramount.
- Quality Control and Vendor Management: Maintaining consistent quality and ensuring adherence to specific company standards and regulatory requirements across diverse outsourced functions necessitates rigorous vendor selection and ongoing performance management.
However, these challenges simultaneously open avenues for growth and innovation. The demand for secure, high-quality, and technologically integrated RAO solutions is driving service providers to invest heavily in advanced security measures and cutting-edge platforms. Furthermore, the burgeoning life sciences sectors in emerging markets, particularly in the Asia Pacific region, offer substantial opportunities for expansion due to favorable operational costs and a growing talent pool.
Market Segmentation Highlights:
According to Prophecy Market Insights, the RAO market is segmented across various dimensions:
- By Service Type: Key segments include Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Applications, Regulatory Submissions, and Regulatory Operations.
- By Indication: Significant therapeutic areas such as Oncology, Neurology, Cardiology, and Immunology drive demand for specialized regulatory expertise.
- By Product Stage: Services are critical across Preclinical, Clinical, and Post-Market Approval (PMA) stages of product development.
- By End-Use: Pharmaceutical Companies, Biotechnology Companies, and Medical Device Companies are the primary end-users of RAO services.
Regional Dynamics: Asia Pacific Leading the Charge:
While North America and Europe continue to hold substantial market shares due to well-established healthcare industries and stringent regulatory environments, the Asia Pacific region is projected to exhibit the highest growth rate. This acceleration is fueled by lower operational costs, a large pool of skilled professionals, and increasing investments in life sciences R&D and manufacturing in countries like India and China.
Competitive Landscape:
The RAO market features a mix of prominent Contract Research Organizations (CROs) and specialized regulatory consulting firms. Key players include Parexel International, IQVIA, Syneos Health, ICON plc, Charles River Laboratories, Genpact, and Freyr. These companies are actively pursuing strategies such as strategic partnerships, mergers and acquisitions, and the expansion of their service portfolios to meet the evolving demands of their global clientele.
The Road Ahead:
The Regulatory Affairs Outsourcing market is on a trajectory of sustained growth, poised to play an increasingly vital role in the global life sciences ecosystem. As regulatory frameworks continue to evolve and companies seek greater efficiency and specialized expertise, the demand for sophisticated RAO solutions will only intensify, solidifying its position as an indispensable partner in bringing safe and effective products to market.
Author:
Authored by Shweta.R, Business Development Specialist at Prophecy Market Insights. This comprehensive analysis is grounded in an extensive blend of primary interviews, industry expert consultations, and in-depth secondary research. It provides strategic insights into the evolving dynamics, competitive landscape, and emerging opportunities within the Regulatory Affairs Outsourcing Market.
About Us:
Prophecy Market Insights is a leading provider of market research services, offering insightful and actionable reports to clients across various industries. With a team of experienced analysts and researchers, Prophecy Market Insights provides accurate and reliable market intelligence, helping businesses make informed decisions and stay ahead of the competition. The company’s research reports cover a wide range of topics, including industry trends, market size, growth opportunities, competitive landscape, and more. Prophecy Market Insights is committed to delivering high-quality research services that help clients achieve their strategic goals and objectives.
Contact Us:
Prophecy Market Insights
Website- https://www.prophecymarketinsights.com