Ever wondered what happens to your cells when you drink a ton of water or eat something super salty? It all comes down to the difference between hypertonic vs hypotonic solutions. These two terms get tossed around a lot in science class, but they actually show up in everyday life too. Basically, it’s about how much stuff—like salt or sugar—is dissolved in a liquid compared to what’s inside your cells. This difference can make your cells either shrink up or puff out like balloons. Let’s break down what makes a solution hypertonic or hypotonic, and why it matters for your body.
Key Takeaways
- Hypertonic solutions have more dissolved stuff (like salt) than the inside of cells, causing water to leave the cells and making them shrink.
- Hypotonic solutions have less dissolved stuff than the inside of cells, so water moves into cells and they swell up.
- Our bodies use these differences to keep things balanced—like in blood, muscles, and kidneys.
- Hypertonic and hypotonic solutions are used in real life, from sports drinks to preserving food and in medical treatments.
- Picking the right type of solution is important for health, because big changes in cell water can be dangerous.
Scientific Basis of Hypertonic vs Hypotonic Solutions
Understanding the difference between hypertonic and hypotonic solutions is actually super useful, whether you’re learning about biology, medicine, or even food prep. Let’s break it down step by step.
Understanding Solute Concentration and Osmosis
Osmosis is just water moving through a membrane from a place with less stuff (solute) to a place with more stuff. Here’s what that means:
- A solute is basically anything dissolved in water, like salt or sugar.
- Hypertonic means a higher concentration of solute compared to another solution.
- Hypotonic means a lower concentration of solute compared to another solution.
For example, if you put a cell in pure water (which has no solute), that’s a hypotonic situation for the cell—water rushes in. If you put the cell in saltwater, it’s hypertonic, and water leaves the cell to balance things out. If you want more details, describing this tonicity comparison can be similar to comparing desktop management models—it’s all about the balance.
Roles of Semi-Permeable Membranes
Cells have a thin outer shell called a semi-permeable membrane. This lets water through but holds back bigger molecules. The semipermeable membrane helps:
- Keep out harmful substances
- Allow water to pass and adjust cell volume
- Make sure the right things stay inside the cell
This is why water moves in or out, depending on what’s outside the cell. So if you’re soaking fish in salty water, microbes in the fish dry out because water rushes out through this membrane.
How Tonicity Differs from Osmolarity
People mix these up all the time. Though they sound close, they aren’t exactly the same. Here’s a quick table to make things clearer:
| Term | What It Measures | Main Focus |
|---|---|---|
| Osmolarity | Total solute per liter | All dissolved particles |
| Tonicity | Solute affecting cells | Swelling or shrinking |
- Tonicity is about what a solution does to a cell—will the cell shrink, swell, or stay the same?
- Osmolarity is about counting all particles in the liquid, no matter their effect on water flow or the cell.
- A solution can be high in osmolarity, but still be isotonic if the solutes don’t cross the membrane and water doesn’t rush in or out.
So to sum up: tonicity is the practical side—what actually happens to your cells—while osmolarity is like the total tally of dissolved bits, regardless of impact.
Effects of Hypertonic vs Hypotonic Environments on Cells
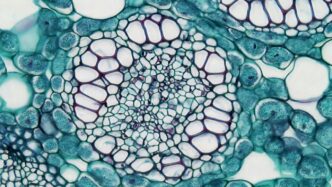
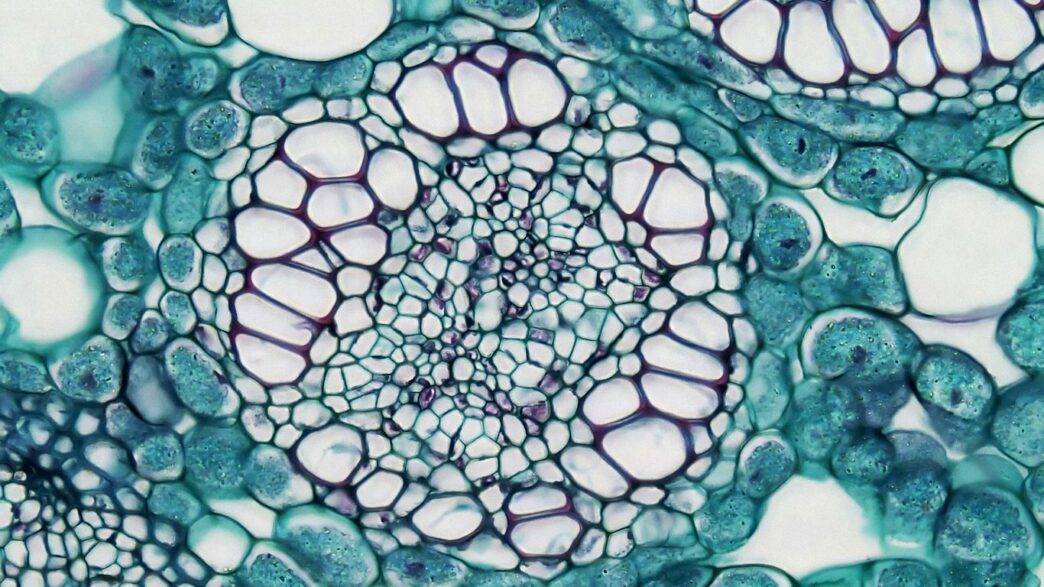
Cell Shrinking and Swelling Mechanisms
When a cell is put in a hypertonic solution, water moves out of the cell to balance things out. This causes the cell to shrink, kind of like a grape turning into a raisin. On the other side, in a hypotonic solution, water rushes inside the cell, and it swells—sometimes even bursting, like popping a balloon. This water movement is just osmosis at work, following the differences in solute concentration on each side of the membrane.
Here’s a quick breakdown:
| Environment | Water Movement | Cell Effect |
|---|---|---|
| Hypertonic | Out of cell | Shrink/crenation |
| Hypotonic | Into cell | Swell/lysis |
Molecular and Cellular Adaptations
Cells don’t just sit back and let water changes happen without trying to do something about it. In hypertonic environments:
- They might make more aquaporin channels to help regulate water flow out.
- Cells can pump in specific organic molecules (like taurine, inositol, or betaine) called osmolytes to balance out water loss.
- Genes for different transporters and proteins switch on or off, basically helping the cell cope with extra or low salt.
Hypotonic solutions usually force cells to get rid of solutes or slow down their water intake so they don’t burst. This is all about balance and making the best out of a tough situation smart practices.
Implications for Cell Survival
A cell’s ability to survive in extreme environments depends a lot on how fast and well it adapts:
- Too much shrinking (in hypertonic surroundings) can mess up protein activity and cell machinery, maybe even causing cell death.
- Swelling from a hypotonic solution may break the plasma membrane, which is a death sentence for most animal cells.
- Some specialized cells—like those in the kidneys—are better equipped for extreme osmotic changes, but most cells don’t handle it well.
In the end, if the environment keeps changing, and cells can’t adapt, survival chances drop quickly. Cellular adjustments work for a short while, but if the challenge sticks around, cells can be overwhelmed and die off.
Hypertonic vs Hypotonic in Human Physiology

When you think about fluids in the body—blood, sweat, or what’s inside your cells—understanding hypertonic and hypotonic solutions explains a lot of what’s going on beneath the surface. Here’s how these types of solutions play out in human physiology.
Responses in Blood and Body Fluids
The balance of solutes in the blood and body fluids is carefully regulated to keep everything running smoothly. Here’s what happens when that balance gets shifted:
- In a hypertonic environment (more solutes outside the cell than inside), water leaves the body’s cells. This causes them to shrivel or shrink, which messes up normal cell functions.
- When your blood is exposed to a hypotonic solution (fewer solutes outside than inside), water rushes into cells, making them swell and potentially burst.
- Everyday examples: drinking distilled water gives the body a hypotonic jolt, while drinking something like beer with more solutes acts as a hypertonic solution compared to your blood flexibility and a vast selection.
Quick Comparison Table
| Type of Solution | Relative Solute Outside Cell | Water Movement | Cell Response |
|---|---|---|---|
| Hypertonic | Higher | Out of cell | Shrinks |
| Isotonic | Equal | None | Stays the same |
| Hypotonic | Lower | Into cell | Swells/Bursts |
Impact on Muscle and Nerve Function
Your muscles and nerves are sensitive to changes in fluid balance:
- Muscle cells: When exposed to a hypertonic solution, water loss leads to cramping and decreased strength, since the cells literally shrink and lose their "juice."
- Nerve cells: They become more irritable or, sometimes, unable to transmit signals if shifting too fast between these fluid environments. Swelling can cause headaches and confusion; shrinkage can slow neural activity.
- In both cases, the disruption is felt quickly because of how fast water can move in or out of these cells.
Adaptation Mechanisms in the Kidneys
The kidneys are basically the body’s fluid and salt managers. They help handle changes in tonicity in a few neat ways:
- When fluids get too hypertonic, osmoreceptors in your brain sense the change. This triggers your thirst and a release of antidiuretic hormone (ADH), which helps you conserve water.
- The kidneys dial up water reabsorption, concentrating the urine so you lose less water and bring your blood back toward a normal range.
- When you drink too much water or take in a hypotonic load, the kidneys flush out extra water to avoid overfilling your cells—that way, cells don’t swell up and burst.
The constant work of these processes means our bodies can handle a variety of fluid types—from pure water to salty snacks—without breaking down. Still, things can get thrown off if changes happen too quickly or go on too long. That’s why understanding how hypertonic and hypotonic solutions work really matters when it comes to health, muscle power, and nerve function.
Practical Examples and Applications of Hypertonic vs Hypotonic Solutions
Let’s be real, these are more than just science class buzzwords—they show up in stuff we use, food we eat, and in medicine all the time. Here’s how hypertonic and hypotonic solutions matter in regular life, medicine, and industry.
Everyday Fluids and Beverages
Most of us don’t think about solute concentration when we pick out what to drink, but it actually has an impact on our bodies.
- Hypotonic drinks, like plain water or weak tea, have less solute than your body fluids. These are best for quick hydration because your cells absorb the water fast.
- Hypertonic drinks, such as fruit juice, sodas, and even beer, have more solute than your blood. If you chug these, water can leave your cells, making you feel even thirstier if you’re already dehydrated.
- Isotonic drinks, like some sports drinks, aim to match your body’s solute levels. These help replace fluids without causing cells to swell up or shrink.
Here’s a quick table comparing some common drinks:
| Solution Type | Example Drinks | Effect on Cells |
|---|---|---|
| Hypotonic | Water, weak tea | Cells swell |
| Isotonic | Sports drink (balanced) | No volume change |
| Hypertonic | Juice, lemonade, beer | Cells shrink |
Medical Uses in Hydration and Rehydration
Doctors and nurses rely on the science of tonicity every day, especially when someone is sick or dehydrated. Picking the right IV solution can mean the difference between helping or making things worse.
- Isotonic saline (0.9% NaCl) is the go-to for most people who just need fluid replacement, like after surgery or lots of sweating.
- Hypotonic IV fluids (like 0.45% saline) are used if someone’s cells are dehydrated and need a gentle push of water inside—the medical team has to be careful though, or cells can burst.
- Hypertonic solutions (like 3% saline) are given in special emergencies, like severe low sodium levels, but they can be risky and need close monitoring.
Role in Food Preservation and Safety
When you think about preserving food, it’s all about stopping bacteria, yeast, and mold from growing—and solute concentration makes this happen.
- Hypertonic solutions, loaded with salt or sugar, are classic in preserving food. That’s why pickles, jams, and salted fish last for ages. Microbes lose water in these salty or sweet surroundings and die off.
- Hypotonic solutions don’t work for preserving food, since they would let bacteria thrive by giving them easy water access.
- Besides traditional methods, food companies use these principles to design products that last longer without needing additives.
In short, knowing whether something is hypertonic or hypotonic changes how we treat injuries, pick our drinks, and even keep our food safe at home.
Clinical Relevance and Health Implications of Tonicity Disturbances
When it comes to how fluids and electrolytes affect the body, tonicity really matters. Losing that balance in tonicity—whether it’s due to too much or too little solute—messes with water movement between cells and their surroundings. Let’s break down some of the main issues that can come up when this balance is thrown off.
Disorders Caused by Imbalanced Tonicity
- Hypertonic conditions draw water out of cells, causing them to shrink and possibly lose function.
- Hypotonic states push water into cells, leading to swelling. Sometimes this even causes cells to burst.
- Prolonged or rapid changes in tonicity can result in dangerous symptoms like brain swelling, confusion, seizures, and even death.
Here are a few disorders where tonicity issues show up:
| Disorder | Tonicity Problem | Common Symptoms |
|---|---|---|
| Hypernatremia | Hypertonic | Thirst, confusion, muscle twitching |
| Hyponatremia | Hypotonic | Headache, nausea, seizures |
| Dehydration (severe) | Hypertonic | Dry mouth, fatigue, rapid heartbeat |
Hyperglycemia and Hypernatremia as Causes of Hypertonicity
When sugar (glucose) or sodium levels get too high in the blood, they act as “effective osmoles” and pull water out of the cells, concentrating them too much. For instance:
- Hyperglycemia (often in uncontrolled diabetes) makes the blood hypertonic, thanks to all that extra glucose floating around. This pulls water from cells and can cause dehydration inside tissues, while also increasing urine output.
- Hypernatremia is basically too much sodium in the blood. This condition is often seen when people can’t replace water losses—like elderly folks who can’t feel thirsty, or people with kidney problems.
Both hyperglycemia and hypernatremia can spiral into life-threatening problems if not caught early. A crazy high sodium or glucose level can make the brain cells shrink, leading to confusion, seizures, or even a coma. Here’s a quick breakdown:
| Condition | Main Osmole | Key Complication |
|---|---|---|
| Hyperglycemia | Glucose | Diabetic coma |
| Hypernatremia | Sodium | Neurologic symptoms |
Dangers of Rapid Osmotic Changes in the Body
Changing tonicity too fast can be just as risky as being imbalanced for too long. For example:
- If you fix sodium levels too quickly, your brain cells may not have time to adapt, causing dangerous swelling or shrinking.
- Rapid correction of chronic low sodium (hyponatremia) can cause osmotic demyelination syndrome—a severe brain disorder.
- When treating diabetic emergencies, replacing fluids too quickly can shift water into or out of brain cells, leading to serious complications.
To sum up:
- The body tries to keep tonicity tightly controlled, but disease or improper treatment can disrupt this.
- Symptoms often develop quickly and can affect the brain, muscles, and heart.
- Measuring things like blood sodium, glucose, and urine concentration helps catch these problems early.
With all that in mind, if you’re interested in how these concepts intersect with medical technology, the rise of fintech solutions is starting to shape healthcare payments and access, too.
So, understanding and managing tonicity isn’t just for science geeks—it’s important for anyone who cares about staying healthy, especially when dealing with chronic illnesses or hospitalization.
Comparing Hypertonic, Hypotonic, and Isotonic Solutions
Understanding the difference between these solution types comes down to where the solute is more concentrated and how water moves as a result. Let’s break down what makes each one unique and how that affects the cells sitting in them.
How Isotonic Solutions Maintain Cellular Balance
Cells are happiest when their surroundings are isotonic. This means the amount of dissolved stuff (solutes) in the fluid outside the cell matches what’s inside the cell.
- No net water movement occurs, so cells keep their usual size and shape.
- Most IV fluids used in hospitals, like 0.9% saline, aim to be isotonic to avoid shocking cells.
- Isotonic solutions are preferred when the goal is to just support hydration without causing any drastic water shifts in or out of the cells.
Key Differences in Water Movement
It all comes down to osmosis—the movement of water through a membrane. Here’s a basic table to keep things straight:
| Solution Type | Solute Compared to Cell | Water Movement | Effect on Cell |
|---|---|---|---|
| Hypotonic | Lower | Into the cell | Swells/Bursts |
| Isotonic | Same | None | Stays the same |
| Hypertonic | Higher | Out of the cell | Shrinks/Shrivels |
A few easy points to remember:
- In a hypotonic solution, the cell sucks up water and can burst, like when you soak raw veggies in pure water.
- In an isotonic solution, the cell just does its thing—no drama, no change.
- In a hypertonic solution, water leaves the cell, making it shrivel up—think of raisins made from grapes.
Choosing the Right Solution for Hydration and Therapy
How do you figure out which solution to use?
- If someone is dehydrated and losing water from their cells (like in some diarrheal illnesses), a hypotonic solution might help rehydrate the cells.
- If cells are at risk of bursting or swelling up, doctors stick to isotonic solutions to keep things steady.
- For certain conditions (like reducing swelling around the brain), hypertonic solutions are used to draw water out of cells.
Picking between hypertonic, hypotonic, and isotonic solutions depends on the direction you want water to move—into cells, out of cells, or not at all. Making the wrong choice can cause some serious issues, so it’s a decision that’s never made casually in a healthcare setting.
Conclusion
So, after looking at hypertonic and hypotonic solutions, it’s clear that these terms are all about how much stuff—like salt or sugar—is dissolved in a liquid compared to another. When you put a cell in a hypotonic solution, water moves in and the cell can swell up. In a hypertonic solution, water leaves the cell, and it shrinks. This is just how nature tries to balance things out. You see this in everyday life, from how sports drinks work to why salty foods can preserve meat. Understanding these differences isn’t just for science class—it actually helps explain a lot about how our bodies work and even why certain foods last longer. Next time you drink water or eat something salty, you’ll know there’s a bit of cell science going on behind the scenes.